X-ray
- Normal in stages 0 and I.
- Osteopenia and sclerosis in stage II.
- Subchondral collapse "crescent sign" in stage III.
- Secondary OA in stage IV.
Magnetic resonance imaging (MRI)
- Ischaemic marrow changes are evident before bone changes are apparent.
- Earliest finding:
- T1 images – decreased signal from ischaemic marrow/single band-like area of low signal intensity.
- T2 images a second, high signal intensity line can be found within the line seen on T1 images, believed to represent hypervascular granulation tissue ("double line sign").
- Rarely AVN may be found on histology with a normal MRI.
- 100% sensitivity, 98% specificity.
Shimizu, et al. Prediction of collapse with MRI of AVN of the femoral head. JBJS 1994; 76A.
- Where at least three-quarters of the diameter of the femoral head and at least two-thirds of the major weight-bearing area is involved – 74% had collapsed within 32 months.
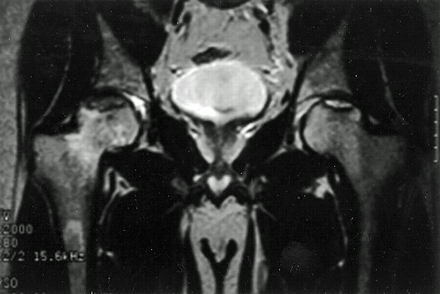
Figure 7. Bilateral hip AVN on coronal MRI.
Bone scan
- Cold area may only be evident in early stages of disease (14–21 days) prior to revascularisation.
- Usually increased uptake at time of study.
- Increased uptake on both sides of joint suggests OA rather than AVN.
- 75–80% sensitivity in precollapse stage.
Single photon emission computed tomography (SPECT) scan
- SPECT imaging is a three-dimensional isotope scanning technique.
- It has been shown to be useful in the analysis of bone graft healing and in the identification of early osteonecrosis.
- Because of its ability to visualise bone reactivity in three dimensions, it is helpful in identifying an area of decreased activity (cold spot) within an area of increased activity.
- This clinical picture can be seen in a very early stage of osteonecrosis.
Classic reference1
Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 1985; 67(1): 3–9.
- Ficat and Arlet proposed the original classification of osteonecrosis in 1964 before the advent of MRI. It consisted of stage I through to stage IV and did not include stage 0.
- This article in 1985 modified the original classification (1964) by including functional exploration of bone and adding a stage 0 previously identified by Hungerford in 1979.2
- Later on at least three further changes to this modified Ficat system have occurred to take account of MRI findings, patient symptoms and modifications of original radiographic findings described.3From having three stages, the system progressed to four stages and then up to six stages, sometimes including symptoms and MRI findings.3Most surgeons still use the simple four-tiered method.
- Despite Mont et al.3identifying at least 16 classification systems in use to grade and describe avascular necrosis, the Ficat system continues to be the most widely used system. One significant change is that histological examination of the hip (biopsy) is no longer needed for a diagnosis of AVN. MRI is the most sensitive and specific diagnostic method.
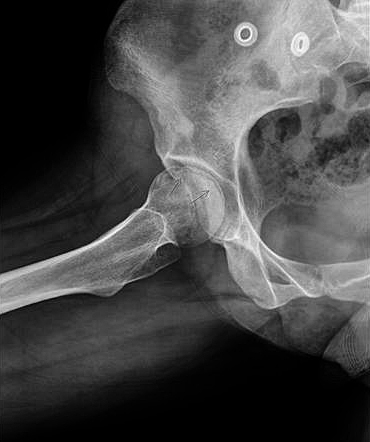
Figure 8. Crescent sign on a lateral hip X-ray
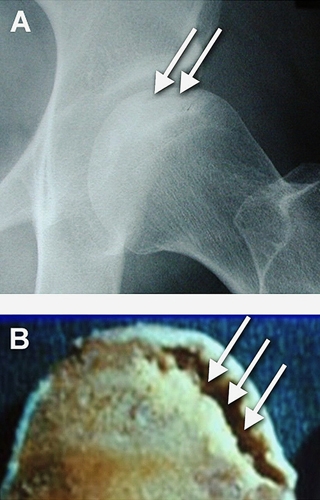
Figure 9. Nontraumatic Osteonecrosis of the Femoral Head
Ficat classification
The Ficat classification is a five-stage system:
- Preclinical: known as a silent hip. No clinical symptoms. Normal radiographs. MRI non diagnostic.
- Pre-radiographic: radiographs usually normal or at most show only minor changes such as subtle loss of clarity with poor definition or blurring of trabeculae pattern. Diagnosed with a positive MRI or bone scan. Earliest clinical manifestation syndrome. Usually presents with sudden onset of hip pain.
- Pre-collapse (before flattening of the head or sequestrum formation): this extends over several months with clinical symptoms and signs persisting or worsening. Radiographs demonstrate osteopenia/sclerosis of the femoral head, spherical head, crescent line due to subchondral fracture.
- Collapse: segmental flattening and collapse of the femoral head. Worsening pain, limp, limited range of motion in all planes.
- Osteoarthritis: terminal phase, secondary degenerative change superimposed on a deformed femoral head.
- Stages 0 to II were described as early stages and stages III and IV were classified as late stages.
Steinberg modification of Ficat classification:4
- Grade 0
- Normal imaging
- Preclinical
- Grade 1
- Normal X-ray
- Early abnormal MRI changes
- Grade 2
- Osteopenia and sclerosis on X-ray
- Grade 3
- Crescent sign
- Grade 4
- Flattening of the femoral head
- Grade 5
- Joint space narrowing
- Grade 6
- Secondary OA
- Stages I–V can be further subdivided into mild (A), moderate (B) or severe (C) to include the extent of femoral head involvement.
Natural history5
- Meta analysis of the literature: 21 studies involving 819 hips, average follow-up 34 months, all treated non-operatively (various protocols of weight-bearing status)
- Rates of preservation of the femoral head:
|
Stage 1
|
35%
|
|
Stage 2
|
31%
|
|
Stage 3
|
13%
|
Treatment6
- Potentially reversible early if corticosteroids or alcohol stopped.
- Symptomatic treatment, weight loss and physiotherapy.
- Bisphosphonates may be of benefit in the pre-collapse stages (controversial).
- Protective weightbearing:
- Start with non-weightbearing with progression to weightbearing allowed as clinical symptoms and signs demonstrate that the hip is less irritable.
- Radiographic and clinical follow-up at 6 week intervals until pain has subsided.
- Success 5–20% at 3–5 years follow-up.
- Core decompression:
- The goal of this procedure is to demonstrate intraosseous venous hypertension and remove a central core of bone from the lesional area effectively to lower intraosseous pressure.
- The biopsy obtained can confirm the disease histologically.
- Patients remain non-weightbearing for 6 weeks post-op.
- There has been substantial controversy as to the effectiveness of this procedure.
- Meta-analysis of 24 studies involving 1206 hips treated with core decompression.
- Rates of preservation of the femoral head:
|
|
Core decomp.
|
No Rx
|
|
Stage 1
|
84%
|
35%
|
|
Stage 2
|
65%
|
31%
|
|
Stage 3
|
47%
|
13%
|
Figure 10. Core decompression
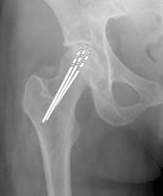
Figure 11. Drill lines demonstrate the pathway of small drill holes used in core decompression
- Electrical stimulation:
- The use of pulsed electromagnetic field therapy with external coils.
- Not commonly used.
- Proximal femoral osteotomy:
- This may compromise later total hip replacement (THR).
- The procedure directly addresses the mechanical aspects of osteonecrosis on the femoral head.
- The goal is preservation of the femoral head by altering the pattern of stress transfer in the diseased head.
- Varus or rotational osteotomies may be used.
Figure 12. Radiograph of proximal femoral osteotomy
- Strut grafting (fibula/tibia/iliac crest)\;
- This procedure, either of the Bonfiglio non-vascularised type or using a vascularised grafting technique, may be used in the pre-collapse stages.
- Cortical strut grafts, e.g. ilium, tibia, fibula, are placed into a core track in the femoral neck under the subchondral bone to help prevent collapse.
- It does not alter the stress-transfer patterns in the upper femoral regions.
- Although early reports of vascularised grafting techniques were encouraging, studies to date are not sufficient to support widespread application(Figure 13).
Figure 13. Vascularised strut grafting
Vascularised pedicle flaps:
- Quadratus femoris graft (metres) – posterior.
- Tensor fascia lata muscle – anterior.
- Rarely used.
Trapdoor procedure:
- Indicated for pre-collapse.
- The surgical procedure includes anterolateral dislocation of the femoral head to expose the area of the femoral head collapse.
- The break in the articular cartilage is identified and opened like a trapdoor.
- Necrotic bone under the flap is excavated and removed.
- This defect is filled with cancellous bone graft from the patient’s iliac crest and overpacked into the defect to prevent subsidence.
- The flap is then carefully repositioned and the hip reduced and the capsule repaired.
- The operation is often followed by an acetabuloplasty with or without a varus femoral osteotomy.
- Good results reported in two studies:
Mont MA, Einhorn TA, Sponseller PD, Hungerford DS. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br 1998; 80(1): 56–62.
Ko JY, Meyers MH, Wenger DR. “Trapdoor” procedure for osteonecrosis with segmental collapse of the femoral head in teenagers. J Pediatr Orthopaed 1995; 15(1): 7–15.
Advanced stages
Arthrodesis:
- More historical, e.g. young patient with unilateral disease.
- 50–80% of cases are, however, bilateral.
THA:
- Both uncemented and cemented total hip arthroplasty have been used in this population.
- Reported success rates are below what appear to be expected from series in other patient populations.
- Results are, however, more predictable in terms of relieving pain and function than many of the other options, and many surgeons will replace the hip if symptoms are severe enough with radiographic collapse.