Modes of bone healing
- Primary bone healing (strain is <2%). Intramembranous healing occurs with absolute stability constructs via Haversian remodeling.
- Secondary bone healing (strain is between 2 and 10%)occurs with non-rigid fixation, as fracture braces, external fixation, bridge plating, intramedullary nailing, etc. via enchondral healing.
- Combination bone healing may occur as a mixture of primary and secondary bone healing depending on the stability throughout the construct.
Table 1. Types of fracture healing based on method of stabilisation
|
Type of immobilisation
|
Predominant type of healing
|
|
|
Cast (closed treatment)
|
Periosteal bridging callus
|
Endochondral ossification
|
|
Compression plate
|
Primary cortical healing (remodeling)
|
Cutting cone type remodeling)
|
|
IM nail
|
Early: periosteal bridging callus (enchondral ossification)
|
Endochondral ossification
|
|
|
Late: medullary callus
|
|
|
External fixation
|
Depends upon the extent of rigidity
|
|
|
Inadequate
|
Hypertrophic non-union
|
Failed endochondral ossification
|
|
|
|
Type II collagen predominates
|
Primary healing
- With primary bone healing there is no movement across the fracture ends under physiological load.
- There is absolute stability with compression that provides a very low strain environment.
- Features include no callus, cutting cones cross the fracture site, direct laying down of new osteones and intramembraneous ossification. It mimics normal bone remodeling.
- For primary bone healing to occur no significant gap at the fracture site (50 micrometres) should be present.
Primary bone formation
- Primary bone formation means that neither connective tissue nor fibrocartilage was present prior to new bone being laid down. There are two types of primary bone healing:
- Gap healing
- Contact healing
- Both types involve a cutting cone that simultaneously resorbs woven bone and replaces it with lamellar bone.
The cutting cone
- Resorption cavities are formed by groups of osteoclasts that have formed a “cutting cone.” This cutting cone advances longitudinally through the new bone in the gap, leaving a resorption cavity.
- The osteoclasts are followed by a thin-walled capillary loop that runs in the centre of the resorption cavity. These vessels are accompanied by mesenchymal cells and osteoblast precursors.
- Newly formed osteoblasts line the resorption cavity and begin producing osteoid.
- Eventually the resorption cavity will fill entirely with connective layers of new bone and become an osteon.
- The synchronised action of both bone-resorption and bone-forming cells results in a regenerating osteon that is capable of advancing in a longitudinal direction parallel to the long axis of bone.
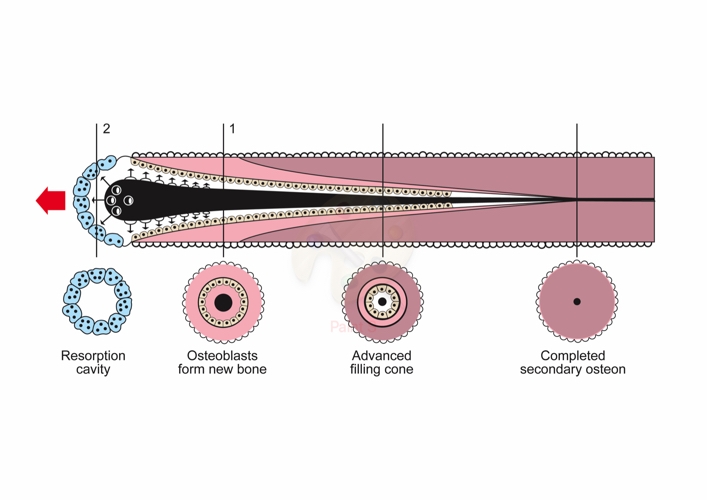
Figure 1. Osteoclastic cutting cone
Gap healing
- Differs from contact healing in that bony union and Haversian remodeling do not occur simultaneously. Anatomical reduction and stable conditions need to be present with a gap less than 1 mm.
- Initial healing occurs with production of woven bone. This is then remodeled via a cutting cone mechanism.
- Haversian remodeling begins with the formation of resorption cavities that penetrate in a longitudinal direction through the necrotic fragment ends and approach the newly formed tissue within the fracture gap.
- The orientation of the new healing bone lamellae and their collagen fibrils differs markedly from their orientation in the fragment ends; it is transverse to the long axis of the diaphysis.
Contact healing
- In contact healing as no gaps are present, Haversian remodeling of the fracture site begins immediately.
Secondary healing
- With secondary fracture healing there is bone formation via tissues that undergo change in material structure until skeletal continuity is restored.
- Cruess and Dumont proposed that secondary fracture healing should be considered to consist of three overlapping phases: (1) inflammation, (2) repair, and (3) remodeling.
- This was expanded by Frost into five stages consisting of (1) haematoma, (2) granulation tissue, (3) callus, (4) modeling, and (5) remodeling.
- In reality although there are a number of different distinct phases of secondary fracture healing described these stages merge into a continuous healing process.

Figure 2. Stages of fracture healing
Table 2. Biochemical steps of fracture healing
|
Step
|
Collagen type
|
|
Mesenchymal
|
I, II, (II. V)
|
|
Chondroid
|
II, IX
|
|
Chondroid-osteoid
|
I, II, X
|
|
Osteogenic
|
I
|
Inflammatory phase (immediate)
- Haematoma formation at the fracture site. Bone is fractured along with a damaged soft tissue envelope that includes periosteum and surrounding muscles with various blood vessels crossing the fracture line ruptured.
- There is an accumulation of haematoma within the medullary canal, between fracture ends and beneath any elevated periosteum. This blood rapidly coagulates to form a clot at the fracture site. Osteocytes deprived of their nutrition die back as far as the junction of collateral channels. The immediate ends of a fracture die. Severely damaged periosteum and marrow as well as other surrounding soft tissues may contribute to necrotic material in this region. The presence of necrotic material elicits an intense acute inflammatory response.
- The haematoma provides a source of haematopoietic cells that secret growth factors and cytokines to promote healing. The key inflammatory cytokines are PDGF, TNF-a, TGF-b, IL-6, IL-1. This leads to vascular ingrowth and cell proliferation, with a migration of acute inflammatory cells (polymorphonuclear leukocytes, macrophages and lymphocytes) to the region. The clotting cascade and the complement system are both activated, which results in the activation of cytokines and signalling molecules that are chemotactic to the inflammatory cells and angiogenic to blood vessels. Bone morphogenic proteins (BMPs) are also released from the damaged bone and are osteoinductive, mitogenic and angiogenic.
- Following the formation of the primary haematoma, a fibrin-rich granulation tissue forms as fibroblasts proliferate. Within this tissue, endochondral formation occurs in between the fracture ends and external to periosteal sites.
- Exudate and necrotic tissue are removed by macrophage phagocytosis. Pluripotent mesenchymal stem cells, osteoblasts and fibroblasts proliferate to produce a new matrix.
- This phase peaks at 48 hours and almost disappears by a week post-fracture. As the acute response subsides, the second phase begins and gradually becomes the predominant pattern.
Repair phase (starts within 2 weeks)
- The haematoma is organised and serves as a fibrin scaffold. There is a change of environment from slightly acidic (acidic tide), to neutral and then slight alkaline (alkaline tide). Osteoblasts need an alkaline environment to lay down bone.
- Pluripotential mesenchymal cells are directly involved in the repair of fractures and form collagen, cartilage and bone. Small variations in their microenvironment determine which behaviour predominates. The manner in which mechanical factors influence fracture healing is explained by Perren’s strain theory (see below). A fracture gap strain of 200% promotes fibroblast proliferation, with fibrous tissue forming in the fracture gap. Less than 15% strain and chondrocytes proliferate laying down collagen matrix and soft callus in the fracture gap. With 2–5% strain osteoblasts start to lay down osteoid that is then mineralised to form hard callus (woven bone).
- Endosteal cells also participate. Surviving osteocytes do not take part in the repair process, because they are destroyed during resorption.
- The majority of cells involved directly in fracture healing enter the fracture site along with granulation tissue, which invades the region with the ingress of capillary buds. Under normal circumstances, the periosteal vessels contribute the majority of capillary buds early in bone healing, with the nutrient medullary artery becoming more important later in the process.
- The cells invade the haematoma and begin to produce callus, which is made up of fibrous tissue, cartilage and young, immature fibre bone. This quickly envelopes the bone ends and leads to a gradual increase in stability of the fracture fragments. The mechanisms that control the behaviour of each individual cell at this stage of the repair process derive from the microenvironment in which the cell finds itself. Compression or the absence of tension discourages the formation of fibrous tissue. Variations in oxygen tension lead to the formation of either bone or cartilage. Cartilage is formed in areas of low oxygen tensions. An environment with a high oxygen tension is beneficial for osteogenic progenitor cell differentiation. Bridging callus (if bone ends are not in continuity) is formed; soft callus initially, later replaced by hard callus (woven bone) via a process of enchondral ossification.
- Early in the repair process, cartilage formation predominates, and glycosaminoglycans (mucopolysaccharides) are found in high concentrations. Later, bone formation is more obvious.
- Hard callus is formed at the periphery by intramembranous bone formation and soft callus is formed in the central region by endochondral ossification. Initially the matrix consists of types I, III and V collagen, glucosaminoglycans and proteoglycans. Some of this collagen is converted to type II and type IX collagen and then type I collagen dominates during osteogenesis, mineralisation and remodeling.
Remodeling phase
- The last phase, which lasts many months, involves stress orientation of disorganised woven bone into hard, dense lamellar bone via cutting cones and is governed by Wollf’s law.
- Osteoclastic resorption of trabeculae occurs, and new struts of bone are laid down that correspond to lines of force. The control mechanism is believed to be electrical.
- The cellular module that controls remodeling is the resorption unit, consisting of osteoclasts, which first resorb bone, followed by osteoblasts, which lay down new Haversian systems. The end result of remodeling is a bone that, if it has not returned to its original form, has been altered so that it may best perform the function demanded of it.