Case based Discusson Tumour Principles
You are on call in a DGH when you are referred a 19-year old traveller who is in ambulatory care with 3 months of a painful swollen lower leg. USS is negative for DVT, but shows a large poorly defined mass. They have performed X-rays which show the following:
Examiner:Describe these x-rays
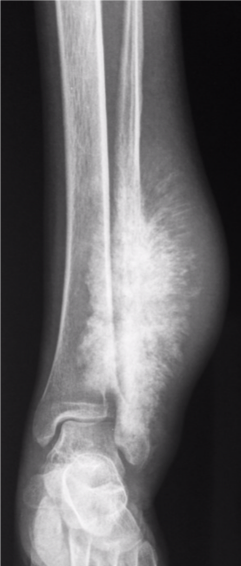
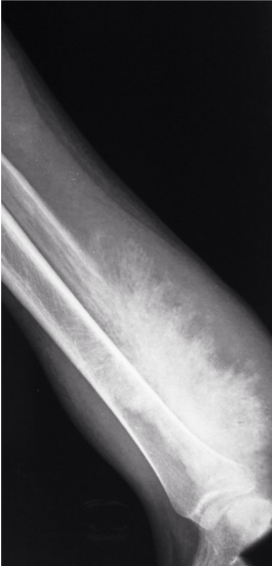
Images courtesy of Dr Aditya Shetty, <a href="https://radiopaedia.org/">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/27260">rID: 27260</a>
Candidate:Poorly defined calcified lesion affecting the left distal third of the tibia and fibula. Wide zone of transition. Sunray spiculation suggestive of extensive soft tissue involvement
Examiner:Is it benign or malignant?
Candidate:This is highly suspicious of a malignant bone tumour
Examiner:What is your understanding of Enneking staging?
Candidate:Enneking staging is commonly used to describe bone tumours, based on histology, site, and metastases. Grade is low (G1) or high (G2). Site is intracompartmental (T1) or extracompartmental (T2). Metastases are not present (M0), or are present (M1).
Examiner:How are you going to maange this patient
Candidate:I would normally refer him onto our local musculoskeletal tumour unit for further management
My understanding of the principles of management of this lesion would be to first stage the tumour
I would want to get more information, so I would take a full history and examination. I would also want to get more imaging of the lesion with an MRI and CT scan
I would attempt to stage the tumour with a CT scan of the chest,abdomen and pelvis